The world is suffering from a silent pandemic of cancers. Cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020, or nearly one in six deaths. The most common cancers are breast, lung, colon and rectum and prostate cancers. (Source: WHO)
While the official benchmark for 2020 was just under 10 million deaths, reliable projections indicate the number has now surpassed 10 million per year and continues to rise. By comparison, cumulative global deaths from COVID from 2020 to 2024 total just over 7 million. Lung cancers are the no. 1 cause of cancer deaths at about double that of colorectal cancers the no. 2 cause of deaths. (Source: DeepSeek)
Traditional cancer treatments including chemotherapy, radiation therapy, nuclear treatments, and surgery, will damage healthy and cancer cells thus posing significant systemic risk. Quality of life of patients is impacted greatly during these traditional treatments. These treatments are costly without government subsidies but provide no assurance of a cure.
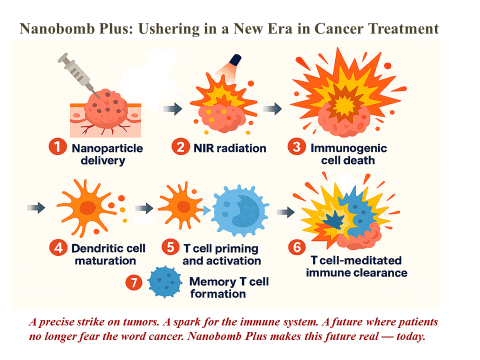
Instead, we believe the ground breaking Nanobomb Plus of Nanotherapy Plus should be the first line of treatment.
Nanobomb Plus can be given as a standalone treatment or in combination with immunotherapy drugs. The objective of Nanotherapy Plus is to improve treatment efficacy and survival outcome through early detection, followed by primary non-toxic therapy of Nanobomb Plus, and secondary immunotherapy to eliminate remaining cancel cells by activating the T-cells to prevent tumour regrowth and to provide ongoing prophylaxis supported by T-cell memory.
Nanobomb Plus is a much improved version of Nanobomb discovered first in 2005 by Dr Balaji (Baloo) Panchapakesan and the University of Delaware. Since we partnered with Dr Baloo the inventor of Nanobomb 1 1/2 years ago, the original Nanobomb has been much improved resulting in our patent pending Nanobomb Plus. Application of nano-engineering as treatment for cancers is gaining traction globally except that Nanobomb Plus is decades ahead of the curve.
In 2005, an in-vitro study of the original Nanobomb was conducted against BT 474 breast cancer cells at the University of Delaware by our inventor partner Dr Baloo, the Chief Investigator of the project. All breast cancer cells were destroyed. This study was funded by US Department of Defence.
With Nanobomb Plus, we envision a future where cancer tumours are shattered precisely at the source, without harming healthy tissues or debilitating side effects. Nanobomb Plus provides a currently unmet clinical need. This is Innovation through transformation to fight cancer.

Together with Dr Baloo, Go-AI has commenced an equal 50/50 partnership Nanotherapy Plus Pty Ltd. Go-AI is the primary funder of Nanotherapy Plus with equity convertible loan. Go-AI will ultimately become the parent entity of Nanotherapy Plus upon its stock exchange listing.
Baloo is a professor, scientist, inventor, innovator, and compassionate leader. He was a professor of mechanical engineering in the United States. His research involves combining nanoscience with medicine. He has published over 100 articles in leading journals and conferences. In 2022, the US State Department awarded him the Fulbright Academic and Professional Excellence Award for his work on breast cancer.
Our business plan includes a “Test to Treat” Strategy to provide a comprehensive solution of early detection via our patent pending Liquid Biopsy Tracker diagnostic tool + Nanobomb Plus therapy + immunotherapy Checkpoint Inhibitors.
Nanotherapy Plus has negotiated very favourable preclinical cost quotes with Hong Kong Science and Technology Park Lab to conduct a preclinical mouse study so as to generate sufficient data to be owned by the company to qualify for human phase 1+2 clinical trials at the Hong Kong University Clinical Trials Centre. Larger scale clinical trials will later be conducted by HKUCTC in conjunction with its network of hospitals in China. Both HKSTPL and HKUCTC are accredited by the China FDA. Results from the preclinical study and the clinical trials will enable the Nanotherapy Plus cancer diagnostic and treatment technologies to be introduced into the China oncology market as well as gaining much visibility with major pharmaceutical companies, thus opening up many more partnership opportunities to accelerate commercialization of Nanotherapy Plus’s diagnostic and treatment solutions.
The following illustrates the DCF valuation of Nanotherapy Plus at various discount rates, projected cumulative gross global income of the company and what global licensee(s) can expect to share of cumulative income for the first 5 years.
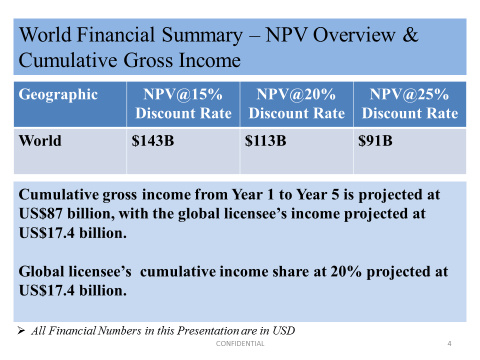
We estimate that at 51% interest of Nanotherapy Plus, Go-AI is valued at A$650 per share. Valuation of Nanotherapy Plus. The funding mechanism provided by Go-AI to Nanotherapy Plus will eventually lead to a greater majority interest of Nanotherapy Plus by Go-AI.
We are seeking professional and sophisticated investor interest in A$27 million by private placement of Go-AI shares to cover the costs of preclinical and phase 1+2 clinical studies as well as cost of stock exchange listing expected In first half of 2026.
For detailed information on Go-AI and the proposed investment, please refer to Home (go-ai.com.au), and Proposed Investment.
Disclaimer:
This document, including the financial model where provided, is provided for general information purposes only and does not take into account each person's individual circumstances. Accordingly, no person should rely or act on the contents of this document, without first obtaining advice from a qualified professional person. For the avoidance of doubt, this document, its author nor Go-AI Pty Ltd and Nanotherapy Plus Pty Ltd and their associates’ related bodies corporate and their respective officers and employees should not be regarded as providing investment advice.